Background: Achieving and sustaining minimal disease negativity improves clinical outcomes in newly diagnosed transplant eligible myeloma (NDMM). Outcomes in NDMM are influenced by levels of minimal residual disease (MRD) post therapy and genetic risk by FISH or molecular profiling prior to therapy. Trials exploring response adapted post transplant strategies using MRD as a prognostic tool are going. We provide an update of the current UK-MRA RADAR study.
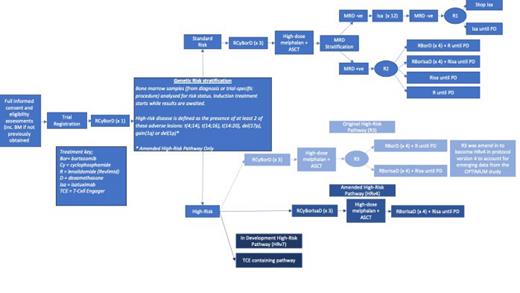
Study Design/Methods: The UK-MRA RADAR study is a national multicentre risk adapted response guided multiarm multistage (MAMS) phase II/III trial which will recruit 1400 patients with NDMM eligible for ASCT. Eligible patients receive induction with 4 cycles of RCyBorD (lenalidomide, cyclophosphamide, bortezomib, dexamethasone), followed by high-dose melphalan and stem cell rescue. Patients with high-risk disease receive Isatuximab in addition to RCyBorD during induction. Genetic risk classification is performed using standard of care FISH testing at local sites. Participants enter the standard or high risk pathway based on presence of atleast two markers: t(4;14), t(14;16), t(14;20), del(17p), del(1p) and gain (1q21). MRD analysis in RADAR uses a multiparametric flow cytometry panel with CD319 and Cd229 used to augment post-isatuximab sample analysis at a central laboratory, with negativity defined at threshold of 10 -5(Figure 1). For standard risk patients, the study addresses two pivotal MRD-related questions: 1) For those with sustained MRD negativity after 12 months of isatuximab maintenance, can treatment be stopped safely to allow patients a treatment-free interval thereby reducing treatment burden? 2) For patients with persistent detectable disease after ASCT, does intensification of consolidation and maintenance therapy augment conversion to MRD negativity compared to lenalidomide maintenance?
High risk participants receive intensified consolidation and maintenance regimens post_ASCT aligned to OPTIMUM/MUKnine ( NCT03188172). A further amendment is planned to explore the activity of a BCMA bispecific antibody in high risk patients pre and post transplant. It is hypothesised this will improve both depth (MRD negativity) and ability to sustain response (PFS). Feasibility and benefits of multimodal MRD incorporating imaging and blood-based testing will be tested in the new high risk pathway. In RADAR, we will also gather information on how patients respond to receiving information about their genetic risk assignment and treatment response (MRD). This is a unique opportunity to understand the patients perspective on personalised medicine, which has implications for implementation in clinical care, and future trial design.
Trial Progress. RADAR opened to recruitment in May 2021 and is currently recruiting across 87 hospital sites with a further 16 sites in set up. 504 patients have been recruited; 87 are classified as high risk on FISH analysis ( 375 standard risk, 15 unable to determine, 19TBC, 8 withdrawn before FISH analysis). 220 have reached D+100 MRD assessment. Trackable phenotypes were available in 98.2% of patients.
Discussion: The RADAR study simultaneously tests escalation and de-escalation strategies, according to disease response, and using MRD to guide post transplant therapy in NDMM patients. Use of central flow cytometry laboratory for MRD has proven feasible in patients to date. Recruitment is on target and outcomes will provide RCT-level evidence for the adaptive use of MRD in treatment allocation and intensification post transplant. The platform design enables adaptation with ability to test new strategies for high risk patients and for standard risk patients who are MRD positive post-transplant.
Funding
This trial is funded by Cancer Research UK (C9203/A24078), Sanofi Genzyme and Celgene : A BMS company. Sanofi Genzyme and Celgene: A BMS company provide study drug and unrestricted educational grants to support study coordination. This work is also supported by Core Clinical trials unit infrastructure from Cancer Research UK ( C7852/A25447). The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the abstract
OffLabel Disclosure:
Ramasamy:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Recordati: Honoraria; BMS ( Celgene): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cairns:Sanofi: Research Funding; Celgene BMS: Honoraria, Research Funding; Janssen: Honoraria; Amgen: Research Funding; Takeda: Research Funding. Cook:Karyopharma: Consultancy; Sanofi: Consultancy; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy. Chapman:Celgene (BMS): Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Popat:GSK: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria. Jackson:Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J&J: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Celgene BMS: Consultancy, Honoraria, Speakers Bureau. Kaiser:Pfizer: Consultancy; GSK: Consultancy; Regeneron: Consultancy; Janssen: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Seagen: Consultancy; Karyopharm: Consultancy. Parrish:BMS Celgene: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Everything Genetic: Consultancy; Janssen: Speakers Bureau; Jazz: Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria. Smith:Janssen, Abbvie, Takeda, BMS, Menarini, Sanofi: Honoraria, Other: Support for conference travel and attendance. The research funding referred to above is agreed inprinciple but has not yet been received and may not be until 2024., Patents & Royalties: Honoraria for speaking, assiting in training events, Research Funding. Jenner:Janssen, BMS, Pfizer, Sanofi: Consultancy, Honoraria.
Isatuximab for newly diagnosed myeloma in induction consolidation and maintenance therapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal